The Ask: $6M to Execute Dual-Track FDA SaMD + CE Mark (EU) Pilots
We are raising $6M in seed funding to execute parallel regulatory pilots in the world's two largest healthcare markets:
| Investment | Market | Objective |
|---|---|---|
| $3M | United States | FDA SaMD approval via Medical Alley (Minnesota) partnerships and leveraging the Harvard Medical School Network |
| $3M | European Union | CE Mark certification through Swiss/German regulatory pathways and clinical validation sites |
Business Model: A Multi-Layered Revenue Strategy
We generate revenue at every layer of the ecosystem, from one-time integration fees to high-margin, recurring software & data licenses:
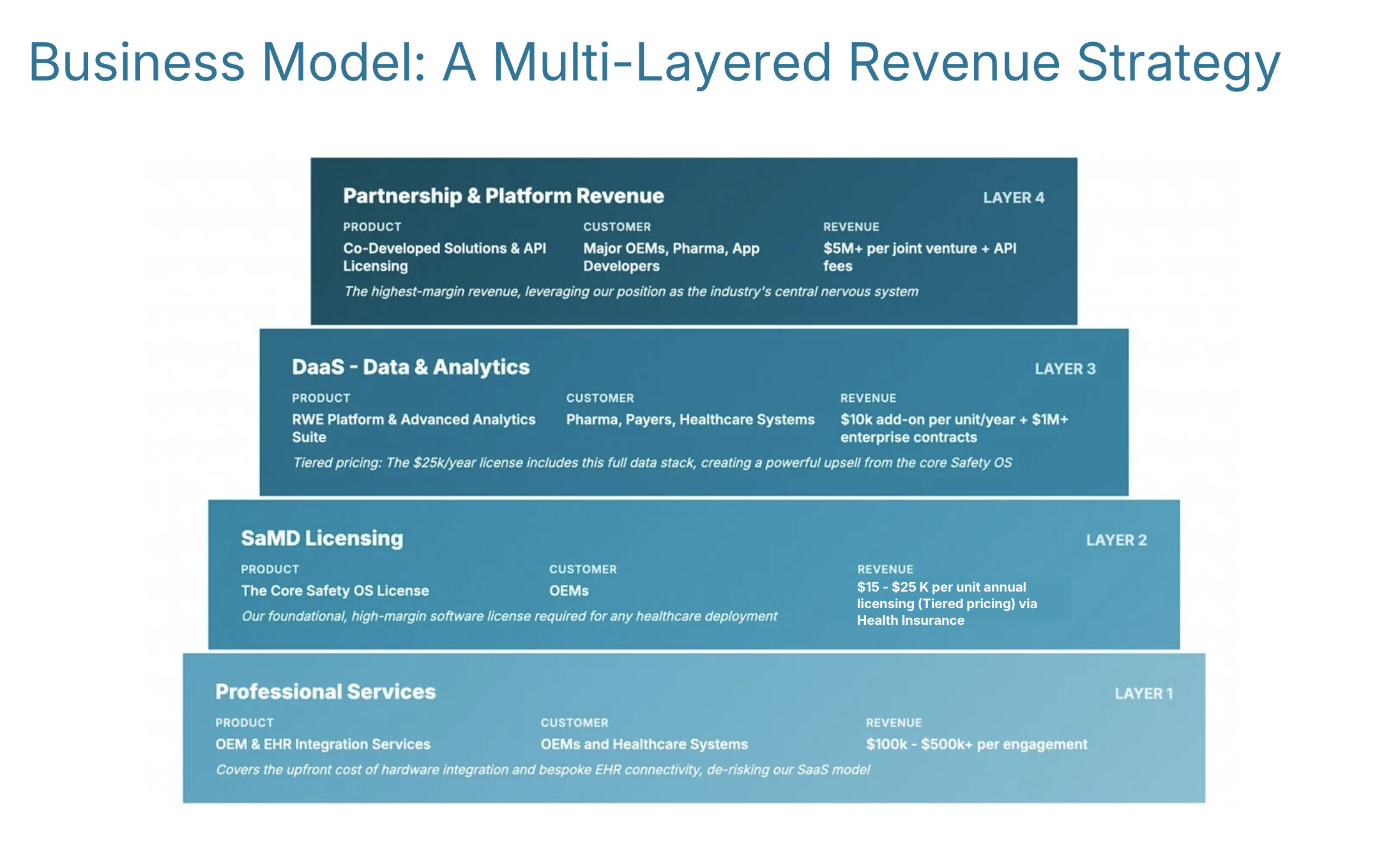
Professional Services (Layer 1)
OEM & EHR Integration Services — Custom integration for robotics manufacturers (Unitree, Boston Dynamics, etc.)
SaMD Licensing (Layer 2)
The Core Safety OS License — Per-robot annual subscription for FDA-compliant software (tiered pricing via Health Insurance)
DaaS - Data & Analytics (Layer 3)
RWE Platform & Advanced Analytics Suite for Pharma, Payers, Healthcare Systems
Tiered pricing: The $25K/year license includes this full data stack, creating a powerful upsell from the core Safety OS
Partnership & Platform Revenue (Layer 4)
Co-Developed Solutions & API Licensing for Major OEMs, Pharma, App Developers
The highest-margin revenue, leveraging our position as the industry's central nervous system
Financial Projections
This capital-efficient model positions us to capture significant value in the emerging humanoid healthcare market.